On behalf of the European Commission, we have received the request below, which we would like to pass on to you. If you currently have 3D printing capacity available, please contact This email address is being protected from spambots. You need JavaScript enabled to view it. urgently so that we can arrange contact.
This example from Italy shows how you can save lives with your contribution from the 3D printer: http://additivemanufacturing.global/index.php/en/news/4519-isinnova-3d-prints-venturi-valves-to-help-coronavirus-patients
„There is an urgent need for masks (see also open source for printing masks) and 25.000 ventilators are needed for respirators, they can be 3D printed, see good example below. You might have these fablabs in your clusters which could be activated to work directly with the hospitals. Or to go on production, if so please let me know and we need to see how we can support this.”
Link to the above mentioned mask: https://www.opensourcemask.com/en/
Here you will find further information on medical standards and requirements (PDF).
* Possible exception:
In duly justified cases, the competent authorities of Member States may decide, within their territory, to authorise the placing on the market and putting into service of devices for which the relevant EU conformity assessment procedure(s) laid down in Directive 93/42/EEC on medical devices have not been carried out and the use of which is in the interest of protection of health.
Tenderers may therefore consider the submission of a separate tender bid for such devices, in particular where they can demonstrate, at the time of submission, that the concerned devices have been authorised in accordance with the conformity assessment procedures established under the national laws of at least one other third country.
Tenderers should note that it is the prerogative of each Member State to decide whether the placing on the market and putting into service of devices referred to above may be authorised on duly justified grounds.
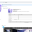
Lot 2 – Ventilators II – invasive
Ventilators under this lot comply with the following minimum quality requirements: