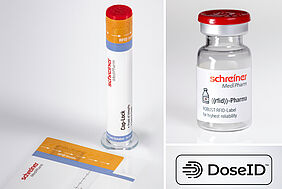
 RFID technology is on the rise in the healthcare sector. But there is a lack of uniform tools to track medicines, devices and consumables. In order to introduce an industry-wide standard, the US consortium "DoseID" was founded - the first industry association for the standardised use of RFID tags in healthcare. Schreiner MediPharm has now joined this consortium of leading companies in the healthcare sector.
RFID technology is on the rise in the healthcare sector. But there is a lack of uniform tools to track medicines, devices and consumables. In order to introduce an industry-wide standard, the US consortium "DoseID" was founded - the first industry association for the standardised use of RFID tags in healthcare. Schreiner MediPharm has now joined this consortium of leading companies in the healthcare sector.
The goal of DoseID is to ensure the interoperability, quality and performance of RFID-tagged products as they move through the pharmaceutical supply chain. The drugs are tracked through all hardware and software systems - from the manufacturer to the retailer, to the hospital, to the patient.
The tracking of a drug can be successful by serialising the drug, container and device. In addition, the RFID tags must function reliably in all IT systems of hospitals and healthcare systems so that the products can be recorded at unit level and tracked throughout their entire life cycle. RFID tags by Schreiner MediPharm make a decisive contribution to this: They can be easily integrated into the pharmaceutical packaging line and smoothly processed there, enable efficient process automation and increase drug and ultimately patient safety.
"As a long-standing supplier of customer-specific RFID labels for the healthcare industry, we consider the need for interoperability and quality standards essential to realize the full potential of RFID. We look forward to being part of the DoseID consortium to jointly drive RFID-based intelligent solutions to improve the pharmaceutical supply chain," comments Stefan Wiedemann, Senior Director Strategic Marketing and Business Development at Schreiner MediPharm.
The unit-level serialization enabled by DoseID goes beyond the requirements of the Drug Supply Chain Security Act (DSCSA), a standard for securing the U.S. supply chain of prescription drugs. To ensure that the standards set by the consortium are met and meet the requirements of pharmaceutical manufacturers, compounding pharmacies, pharmacy automation service providers and manufacturers of RFID inlays and tags, a special RFID tag certification will also be awarded following third-party testing.
www.schreiner-group.com